All About Subatomic Particles
·
Try some
practice worksheets
How to find the number of
protons, neutrons, and electrons that atoms have, as well as finding the number
of valence electrons.
|
Finding Protons, Neutrons, and Electrons of Elements One of the things students frequently have problems with is determining how many protons, neutrons, and electrons an element has, based only on some sparse information given to you on the periodic table or in a problem. This tutorial will give you everything you need to figure it out. First, let's examine one of the entries in the periodic table:
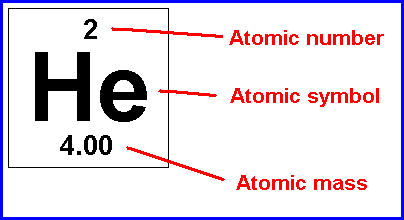
This is what the entry for helium is on a normal periodic table. The atomic number is found at the top, and is equal to the number of protons that an element has. The reason that all atoms of the element have the same number of protons is that the number of protons determines what element is present - if there were a different number of protons, you would have a different element. For neutral atoms, the atomic number is also equal to the number of electrons present because the number of electrons equals the number of protons in neutral atoms. The atomic symbol is different for every element. As you might guess, the atomic symbol and atomic number are very closely related to one another. After all, any element that has the atomic number "2" will have the atomic symbol "He". As a result, if you don't know the atomic number of an element, you can look up the atomic symbol on the periodic table to find it. As a resuilt, you can determine the number of protons an element has from the atomic symbol if you have a periodic table handy. The atomic mass is also different for every element, and also for isotopes of the same element. The atomic mass is equal to the number of protons that an element has plus the number of neutrons that an element has. This is because protons have a mass of approximately 1 amu (atomic mass unit) and neutrons have a mass of approximately 1 amu. Electrons don't count in this calculation, because their mass is small enough that it can usually be ignored. So, to recap:
For a worksheet of practice problems of this type, click here. |
|
Finding the number of valence electrons of an element This is easier than you might think. First of all, let's go through a couple of terms: Octet rule: The rule that says that all elements want to have the same electron configurations as the nearest noble gas to them. This is because the noble gases are really stable, and all elements want to be stable in the same way. Elements become like the nearest noble gas by gaining or losing electrons, or by sharing electrons with other atoms. Valence electrons: The number of s- and p- electrons in the outermost energy level. You can find the number of valence electrons by counting backwards from the element you're interested in to the last noble gas. However, and this is important for some elements, when you're counting backwards, you want to skip over the d- and f- parts of the periodic table. For example, sulfur has 6 valence electrons, as does selenium. Why does selenium have six valence electrons when there are more elements betwen it and the last noble gas? It's because we skip over the entire d-block of elements. Of course, anybody who's read this far is probably curious as to why we need to worry about the octet rule or the number of valence electrons. Well, I'm glad you asked. The octet rule allows us to determine the charge that an element will have when it forms ionic compounds. To find this charge, you'll count from the element that you're interested in to the nearest noble gas. If counting backwards takes you to the nearest noble gas (as in the case of lithium and magnesium), the charge is equal to +[however many elements you need to count across]. For example, since you count backwards one element from lithium to reach the nearest noble gas (which is helium), the charge is +1. If counting forwards takes you to the nearest noble gas (as in the case of oxygen and chlorine), the charge is equal to -[the number of elements you need to count across]. For example, since you count forwards two elements from oxgyen to reach neon, the charge of oxygen when it forms ionic compounds is -2. The number of valence electrons allows us to determine how many electrons an element has that can do covalent bonding, and for this reason is extremely important for nonmetals. For example, when oxygen bonds with two hydrogen atoms, we know that our resulting Lewis structure will need to show eight electrons, as oxygen has six valence electrons (determined by counting backwards to He) and the two hydrogens each have one valence electron. For more info on how to find Lewis structures, click here. |
Questions? Comments? Email them to me at misterguch@chemfiesta.com